INTRODUCTION
All amino acids are derived from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. Nitrogen enters these biosynthetic pathways by way of glutamate and glutamine. Some pathways are simple, others are not. Ten of the amino acids are just one or several steps removed from the common metabolite from which they are derived. The biosynthetic pathways for others, such as the aromatic amino acids, are more complex.
Organisms vary greatly in their ability to synthesize the 20 common amino acids. Whereas most bacteria and plants can synthesize all 20, mammals can synthesize only about half of them—generally those with simple pathways. These are often called nonessential amino acids. The label is somewhat misleading, however, because innate biosynthetic pathways often do not provide enough of these amino acids to support optimal growth and health.
The remaining amino acids, the essential amino acids, cannot be synthesized by most animals and must be obtained from food. Unless otherwise indicated, the pathways for the 20 common amino acids presented below are those operative in bacteria. A useful way to organize these biosynthetic pathways is to group them into six families corresponding to their metabolic precursors, and we use this approach to structure the detailed descriptions that follow.
In addition to these six precursors, there is a notable intermediate in several pathways of amino acid and nucleotide synthesis: 5-phosphoribosyl-1- pyrophosphate (PRPP): PRPP is synthesized from ribose 5-phosphate derived from the pentose phosphate pathway, in a reaction catalyzed by ribose phosphate pyro-phosphokinase: This enzyme is allosterically regulated by many of the biomolecules for which PRPP is a precursor.
Given below are Amino Acid Biosynthetic Families, Grouped by Metabolic Precursor


BIOSYNTHESIS FROM ALPHA KETOGLUTARATE
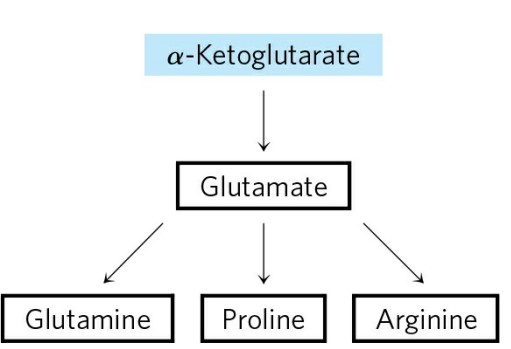
The biosynthetic pathways to glutamate and glutamine are simple, and all or some of the steps occur in most organisms. The most important pathway for the assimilation of into glutamate requires two reactions. First, glutamine synthetase catalyzes the reaction of glutamate and to yield glutamine. This reaction takes place in two steps, with enzyme-bound γglutamyl phosphate as an intermediate.

- Glutamine synthetase is found in all organisms. In addition to its importance for assimilation in bacteria, it has a central role in amino acid metabolism in mammals, converting free , which is toxic, to glutamine for transport in the blood .
- Glutamate can also be formed in yet another, albeit minor, pathway: the reaction of α-ketoglutarate and to form glutamate in one step. This is catalyzed by glutamate dehydrogenase, an enzyme present in all organisms. Reducing power is furnished by NADPH :-

- In the first step of proline synthesis, ATP reacts with the γ-carboxyl group of glutamate to form an acyl phosphate, which is reduced by NADPH or NADH to glutamate γ semialdehyde. This intermediate undergoes rapid spontaneous cyclization and is then reduced further to yield proline.
- Arginine is synthesized from glutamate via ornithine and the urea cycle in animals. In principle, ornithine could also be synthesized from glutamate γ-semialdehyde by transamination, but the spontaneous cyclization of the semialdehyde in the proline pathway precludes a sufficient supply of this intermediate for ornithine synthesis. Bacteria have a de novo biosynthetic pathway for ornithine (and thus arginine) that parallels some steps of the proline pathway but includes two additional steps that avoid the problem of the spontaneous cyclization of glutamate γ-semialdehyde. In the first step, the α-amino group of glutamate is blocked by an acetylation requiring acetyl-CoA; then, after the transamination step, the acetyl group is removed to yield ornithine.
BIOSYNTHESIS FROM 3-PHOSPHOGLYCERATE

- The major pathway for the formation of serine is the same in all organisms. In the first step, the hydroxyl group of 3-phosphoglycerate is oxidized by a dehydrogenase (using NAD+ ) to yield 3- phosphohydroxypyruvate. Transamination from glutamate yields 3- phosphoserine, which is hydrolyzed to free serine by phosphoserine phosphatase.
- Serine (three carbons) is the precursor of glycine (two carbons) through removal of a carbon atom by serine hydroxymethyltransferase. Tetrahydrofolate accepts the β carbon (C-3) of serine, which forms a methylene bridge between N-5 and N-10 to yield N 5 ,N 10 – methylenetetrahydrofolate. The overall reaction, which is reversible, also requires pyridoxal phosphate. In the liver of vertebrates, glycine can be made by another route: the reverse of the reaction shown in, catalyzed by glycine synthase (also called glycine cleavage enzyme)
- Plants and bacteria produce the reduced sulfur required for the synthesis of cysteine (and methionine, described later) from environmental sulfates; the pathway is shown on the right side of. Sulfate is activated in two steps to produce 3′-phosphoadenosine 5′-phosphosulfate (PAPS), which undergoes an eight-electron reduction to sulfide. The sulfide is then used in the formation of cysteine from serine in a two-step pathway.
- Mammals synthesize cysteine from two amino acids: methionine furnishes the sulfur atom, and serine furnishes the carbon skeleton. Methionine is first converted to S-adenosylmethionine, which can lose its methyl group to any of a number of acceptors to form S-adenosylhomocysteine (adoHcy). This demethylated product is hydrolyzed to free homocysteine, which undergoes a reaction with serine, catalyzed by cystathionine α-synthase, to yield cystathionine. Finally, cystathionine γ-lyase, a PLPrequiring enzyme, catalyzes removal of ammonia and cleavage of cystathionine to yield free cysteine.

BIOSYNTHESIS FROM OXALOACETATE

- Methionine, threonine, lysine, isoleucine, valine, and leucine are essential amino acids; humans cannot synthesize them. Their biosynthetic pathways in bacteria are complex and interconnected. Aspartate gives rise to methionine, threonine, and lysine. Branch points occur at aspartate β-semialdehyde, an intermediate in all three pathways, and at homoserine, a precursor of threonine and methionine.
- Threonine, in turn, is one of the precursors of isoleucine. The valine and isoleucine pathways share four enzymes. Pyruvate gives rise to valine and isoleucine in pathways that begin with condensation of two carbons of pyruvate (in the form of hydroxyethyl thiamine pyrophosphate
SYNTHESIS FROM PHOSPHENOLPYRUVTE

- Aromatic rings are not readily available in the environment, even though the benzene ring is very stable. The branched pathway to tryptophan, phenylalanine, and tyrosine, occurring in bacteria, fungi, and plants, is the main biological route of aromatic ring formation. It proceeds through ring closure of an aliphatic precursor followed by stepwise addition of double bonds.
- The first four steps produce shikimate, a seven-carbon molecule derived from erythrose 4-phosphate and phosphoenolpyruvate. Shikimate is converted to chorismate in three steps that include the addition of three more carbons from another molecule of phosphoenolpyruvate. Chorismate is the first branch point of the pathway, with one branch leading to tryptophan, the other to phenylalanine and tyrosine.
- In the tryptophan branch, chorismate is converted to anthranilate in a reaction in which glutamine donates the nitrogen that will become part of the indole ring. Anthranilate then condenses with PRPP. The indole ring of tryptophan is derived from the ring carbons and amino group of anthranilate plus two carbons derived from PRPP. The final reaction in the sequence is catalyzed by tryptophan synthase. This enzyme has an α2β2.
- Subunit structure and can be dissociated into two α subunits and a β2 unit that catalyze different parts of the overall reaction.

- The second part of the reaction requires pyridoxal phosphate. Indole formed in the first part is not released by the enzyme, but instead moves through a channel from the α-subunit active site to one of the βsubunit active sites, where it condenses with a Schiff base intermediate derived from serine and PLP. Intermediate channeling of this type may be a feature of the entire pathway from chorismate to tryptophan.
- Enzyme active sites catalyzing different steps (sometimes not sequential steps) of the pathway to tryptophan are found on single polypeptides in some species of fungi and bacteria, but are separate proteins in other species. In addition, the activity of some of these enzymes requires a noncovalent association with other enzymes of the pathway. These observations suggest that all the pathway enzymes are components of a large, multienzyme complex in both bacteria and eukaryotes.
- Such complexes are generally not preserved intact when the enzymes are isolated using traditional biochemical methods, but evidence for the existence of multienzyme complexes is accumulating for this and other metabolic pathways.
- In plants and bacteria, phenylalanine and tyrosine are synthesized from chorismate in pathways much less complex than the tryptophan pathway. The common intermediate is prephenate. The final step in both cases is transamination with glutamate.
- Animals can produce tyrosine directly from phenylalanine through hydroxylation at C-4 of the phenyl group by phenylalanine hydroxylase; this enzyme also participates in the degradation of phenylalanine. Tyrosine is considered a conditionally essential amino acid, or as nonessential insofar as it can be synthesized from the essential amino acid phenylalanine
AMINO ACID SYNTHESIS

- The pathway to histidine in all plants and bacteria differs in several respects from other amino acid biosynthetic pathways. Histidine is derived from three precursors PRPP contributes five carbons, the purine ring of ATP contributes a nitrogen and a carbon, and glutamine supplies the second ring nitrogen.
- The key steps are condensation of ATP and PRPP, in which N1 of the purine ring is linked to the purine ring opening that ultimately leaves N-1 and C-2 of adenine linked to the ribose and formation of the imidazole ring, a reaction in which glutamine donates a nitrogen.
CONCLUSION
- Plants and bacteria synthesize all 20 common amino acids. Mammals can synthesize about half; the others are required in the diet (essential amino acids).
- Among the nonessential amino acids, glutamate is formed by reductive amination of α-ketoglutarate and serves as the precursor of glutamine, proline, and arginine. Alanine and aspartate (and thus asparagine) are formed from pyruvate and oxaloacetate, respectively, by transamination.
- The carbon chain of serine is derived from 3-phosphoglycerate. Serine is a precursor of glycine; the β-carbon atom of serine is transferred to tetrahydrofolate. In microorganisms, cysteine is produced from serine and from sulfide produced by the reduction of environmental sulfate. Mammals produce cysteine from methionine and serine by a series of reactions requiring Sadenosylmethionine and cystathionine.
- Among the essential amino acids, the aromatic amino acids (phenylalanine, tyrosine, and tryptophan) form by a pathway in which chorismate occupies a key branch point. Phosphoribosyl pyrophosphate is a precursor of tryptophan and histidine. The pathway to histidine is interconnected with the purine synthetic pathway. Tyrosine can also be formed by hydroxylation of phenylalanine (and thus is considered conditionally essential). The pathways for the other essential amino acids are complex.
- Essential amino acids, like nonessential amino acids, are synthesized from familiar metabolic precursors. Their synthetic pathways are present only in microorganisms and plants, however, and usually involve more steps than those of the nonessential amino acids. For example, lysine, methionine, and threonine are all synthesized from aspartate in pathways whose common first reaction is catalyzed by aspartokinase, an enzyme that is present only in plants and microorganisms.
- Similarly, valine and leucine are formed from pyruvate; isoleucine is formed from pyruvate and -ketobutyrate; and tryptophan, phenylalanine, and tyrosine are formed from phosphoenolpyruvate and erythrose-4-phosphate. The enzymes that synthesize essential amino acids were apparently lost early in animal evolution, possibly because of the ready availability of these amino acids in the diet.
Discover more from ZOOLOGYTALKS
Subscribe to get the latest posts sent to your email.

