Introduction – TRYPANOSOMA
Trypanosomiasis, also known as “sleeping sickness,” is caused by microscopic parasites of the species Trypanosoma brucei. It is transmitted by the tsetse fly (Glossina species), which is found only in rural Africa. Although the infection is not found in the United States, historically, it has been a serious public health problem in some regions of sub-Saharan Africa. Currently, about 10,000 new cases each year are reported to the World Health organization; however, it is believed that many cases go undiagnosed and unreported. Sleeping sickness is curable with medication, but is fatal if left untreated.
Classification of Trypanosoma
- Phylum – Protozoa
- Subphylum– Sarcomastigophora
- Superclass– Mastigophora
- Class– Zoomastigophorea
- Order– Kinetoplastida
- Genus– Trypanosoma
- Species– gambiense
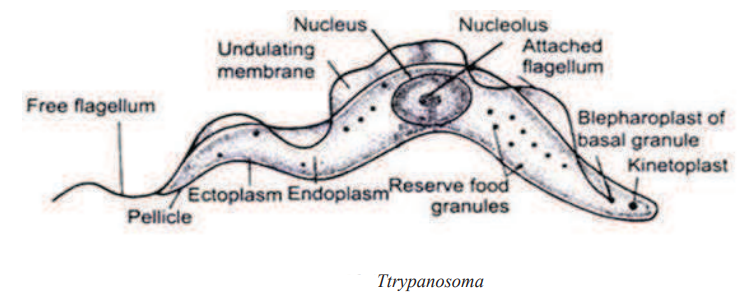
General Characters of Trypanosoma
- It is found mainly in South East Asia, Central & West Africa.
- Trypanosomes are essentially found near moist places.
- Trypanosoma is a monophyletic group of unicellular parasitic flagellate protozoa characterized by possession of a flagellum near posterior end of body.
- Trypanosomes infect a variety of hosts & cause various diseases.
- Polymorphism is shown by trypanosomes on the basis of position of kinetoplast & blepharoplast and the course taken by the flagellum.
- Trypanosomes are heteroxenous i.e. requiring more than one obligatory host to complete its life cycle.
- They are mostly transmitted via a vector.
- Majority of trypanosomes are blood feeding invertebrates.
- Inside an invertebrate host they are generally found in intestine where as in a mammalian host they occupy blood stream.
- It is microscopic, elongated, leaf like tapering at both ends.
- The anterior end contains the free flagellum where as posterior end is blunt.
- Whole body is externally surrounded by thin, elastic covering called pellicle.
- Undulating membrane is an adaptation for locomotion inside the host body.
- It is a membranous fold which is attached to the pellicle originating from the flagellum.
- Cytoplasm is enclosed within the pellicle. Nucleus is large, oval & vesicular within the cytoplasm.
- It reproduces asexually by longitudinal binary fission.
- The long & slender trypanosomes show adaptations to antibodies produced by host body & continue to survive and multiply.
Life Cycle of Trypanosoma
Trypanosoma exhibits polymorphism i.e. occur in four morphological forms which are Leishmanial (Amastigote) form: It is round or oval with a nucleus, blepharoplast & kinetoplast. Flagellum reduced and fibril like embedded in cytoplasm. Leptomonad (Promastigote) form: Body elongated nucleus large & anteriorly located blepharoplast and kinetoplast. Flagellum short & unattached. Crithidial (epimastigote) form: Body elongate, blepharoplast & kinetoplast placed immediately anterior to nucleus. Undulating membrane inconspicuous. Crithidial form is represented only in salivary glands of tse-tse fly. Trypanosomid (Trypomastigote) form: Elongate body with blepharoplast & kinetoplast situated near the posterior end. Undulating membrane conspicuous.
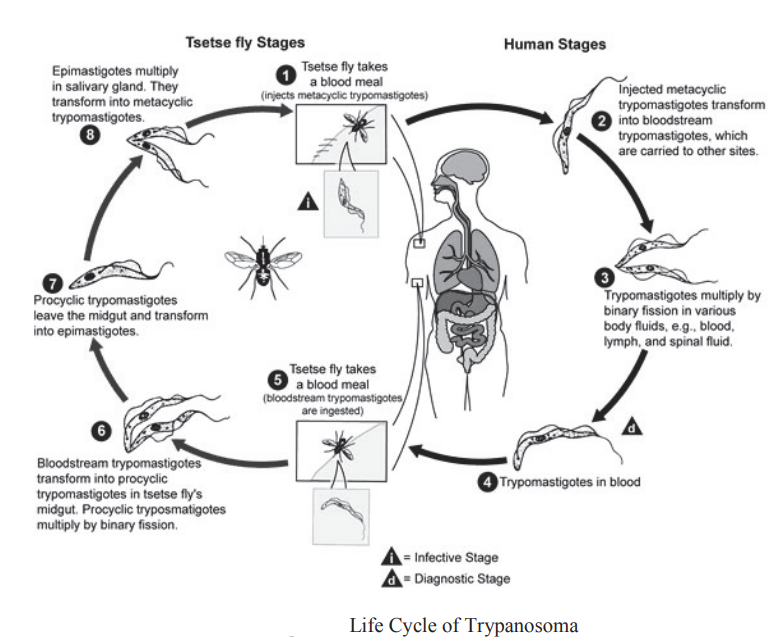
Trypanosoma is digenetic that is completes its life cycle in two hosts. The primary host is man and the secondary host is a blood sucking insect called tsetse fly (Glossina palpalis). The infection to humans by trypanosomes is transmitted by the bite of tsetse fly which contains infective metacyclic forms in salivary glands. The tsetse fly feeding upon mammalian blood releases the contaminated trypanosomes into his blood stream. All stages of trypanosome in human are extracellular i.c. present in the blood plasma.
The vector takes in short stumpy forms along with the blood which cominue to develop inside it into long slender forms & multiply by asexual method. After transformation inside vector they move into its mouth region & metamorphose into crithidial forms having shortened body, reduced flagellum. The crithidial form further transform into slender metacyclic non-motile form which is the infective stage.
During a blood meal on the mammalian host, an infected tsetse fly (genus Glossina) injects matacyclic trypomastigotes into skin tissue. The parasites enter the lymphatic system and pass into the bloodstream. Inside the host, they transform into bloodstream trypomastigotes, are carried to other sites throughout the body, reach other blood fluids (e.g., lymph, spinal fluid), and continue the replication by binary fission. The entire life cycle of African Trypanosomes is represented by extracellular stages. The tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mammalian host.
In the fly’s midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission , leave the midgut, and transform into epimastigotes . The epimastigotes reach the fly’s salivary glands and continue multiplication by binary fission . The cycle in the fly takes approximately 3 weeks. Humans are the main reservoir for Trypanosoma brucei gambiense, but this species can also be found in animals. Wild game animals are the main reservoir of Trypanosoma brucei rhodesiense.
Pathogenecity
Protozoan hemo flagellates belonging to the complex Trypanosoma brucei. Two subspecies that are morphologically indistinguishable cause distinct disease patterns in humans: Trypanosoma brucei gambiense causes West African sleeping sickness and T. b. rhodesiense causes East African sleeping sickness. (A third member of the complex, T. b. brucei, under normal conditions does not infect humans).
Trypanosomes cause Gambian trypanosomiasis /Sleeping sickness in man. The metacyclic promastigote are introduced subcutaneously by the bite of tsetse fly & multiply in 2-3 days. There is itching, swelling, pain & redness developed at the site where vector bites. The earliest sign of generalized infection is loss of weight, fever followed by headache & pain in the joints. 5-12 days after infection the parasite is found in the blood stream swimming freely. Special character of disease is enlargement of cervical lymph nodes called as winterbottom’s sign. The disease sleeping sickness is caused when parasites invade cerebrospinal fluid of central nervous system.
The clinical course of human African trypanosomiasis has two stages. In the first stage, the parasite is found in the peripheral circulation, but it has not yet invaded the central nervous system. Once the parasite crosses the blood-brain barrier and infects the central nervous system, the disease enters the second stage. The subspecies that cause African trypanosomiasis have different rates of disease progression, and the clinical features depend on which form of the parasite (Trypanosoma brucei rhodesiense or Trypanosoma brucei gambiense) is causing the infection. However, infection with either form will eventually lead to coma and death if not treated.
T. b. rhodesiense infection (East African sleeping sickness) progresses rapidly. In some patients, a large sore (a chancre) will develop at the site of the tsetse bite. Most patients develop fever, headache, muscle and joint aches, and enlarged lymph nodes within 1-2 weeks of the infective bite. Some people develop a rash. After a few weeks of infection, the parasite invades the central nervous system and eventually causes mental deterioration and other neurologic problems. Death ensues usually within months.
T. b. gambiense infection (West African sleeping sickness) progresses more slowly. At first, there may be only mild symptoms. Infected persons may have intermittent fevers, headaches, muscle and joint aches, and malaise. Itching of the skin, swollen lymph nodes, and weight loss can occur. Usually, after 1-2 years, there is evidence of central nervous system involvement, with personality changes, daytime sleepiness with nighttime sleep disturbance, and progressive confusion. Other neurologic signs, such as partial paralysis or problems with balance or walking may occur, as well as hormonal imbalances. The course of untreated infection rarely lasts longer than 6-7 years and more often kills in about 3 years.
Diagnosis
The diagnosis of African Trypanosomiasis is made through laboratory methods, because the clinical features of infection are not sufficiently specific. The diagnosis rests on finding the parasite in body fluid or tissue by microscopy. The parasite load in Trypanosoma brucei rhodesiense infection is substantially higher than the level in Trypanosoma brucei. gambienseinfection.
T. b. rhodesiense parasites can easily be found in blood. They can also be found in lymph node fluid or in fluid or biopsy of a chancre. Serologic testing is not widely available and is not used in the diagnosis, since microscopic detection of the parasite is straightforward.
The classic method for diagnosing T. b. gambiense infection is by microscopic examination of lymph node aspirate, usually from a posterior cervical node. It is often difficult to detect Trypanosoma brucei gambiense in blood. Concentration techniques and serial examinations are frequently needed. Serologic testing is available outside the U.S. for T. b. gambiense; however, it normally is used for screening purposes only and the definitive diagnosis rests on microscopic observation of the parasite.
All patients diagnosed with African trypanosomiasis must have their cerebrospinal fluid examined to determine whether there is involvement of the central nervous system, since the choice of treatment drug(s) will depend on the disease stage. The World Health Organization criteria for central nervous system involvement include increased protein in cerebrospinal fluid and a white cell count of more than 5. Trypanosomes can often be observed in cerebrospinal fluid in persons with second stage infection.
Treatment
All persons diagnosed with African Trypanosomiasis should receive treatment. The specific drug and treatment course will depend on the type of infection (T. b. gambiense or T. b. rhodesiense) and the disease stage (i.e. whether the central nervous system has been invaded by the parasite). Pentamidine, which is the recommended drug for first stage T. b. gambiense infection, is widely available in the U.S. The other drugs (suramin, melarsoprol, eflornithine, and nifurtimox) used to treat African trypanosomiasis are available in the U.S. only from the CDC. Physicians can consult with CDC staff for advice on diagnosis and management and to obtain otherwise unavailable treatment drug.
There is no test of cure for African trypanosomiasis. After treatment patients need to have serial examinations of their cerebrospinal fluid for 2 years, so that relapse can be detected if it occurs.
Prevention and Control from Trypanosoma
There is no vaccine or drug for prophylaxis against African trypanosomiasis. Preventive measures are aimed at minimizing contact with tsetse flies. Local residents are usually aware of the areas that are heavily infested and they can provide advice about places to avoid. Other helpful measures include:
- Wear long-sleeved shirts and pants of medium-weight material in neutral colors that blend with the background environment. Tsetse flies are attracted to bright or dark colors, and they can bite through lightweight clothing.
- Inspect vehicles before entering. The flies are attracted to the motion and dust from moving vehicles.
- Avoid bushes. The tsetse fly is less active during the hottest part of the day but will bite if disturbed.
- Use insect repellent. Permethrin-impregnated clothing and insect repellent have not been proved to be particularly effective against tsetse flies, but they will prevent other insect bites that can cause illness.
Control of African trypanosomiasis rests on two strategies: reducing the disease reservoir and controlling the tsetse fly vector. Because humans are the significant disease reservoir for T. b. gambiense, the main control strategy for this subspecies is active case-finding through population screening, followed by treatment of the infected persons that are identified. Tsetse fly traps are sometimes used as an adjunct. Reducing the reservoir of infection is more difficult for T. b. rhodesiense, since there are a variety of animal hosts. Vector control is the primary strategy in use. This is usually done with traps or screens, in combination with insecticides and odors that attract the flies
Discover more from ZOOLOGYTALKS
Subscribe to get the latest posts sent to your email.


